Rotator Cuff Healing Index (RoHI) Risk Factors
Almost 1 in 5 people fail to heal their primary rotator cuff repair. Many factors influence the risk of failure in tendon healing. A 15-point scoring index (RoHI) was developed to predict healing of the rotator cuff and allows for consideration of when to use graft augmentation to improve biomechanical strength and tendon healing.¹
Reference
1. Kwon J, Kim SH, Lee YH, Kim TI, Oh JH. The Rotator Cuff Healing Index: a new scoring system to predict rotator cuff healing after surgical repair. Am J Sports Med. 2019;47(1):173-180. doi:10.1177/0363546518810763
Educational Resources and Products
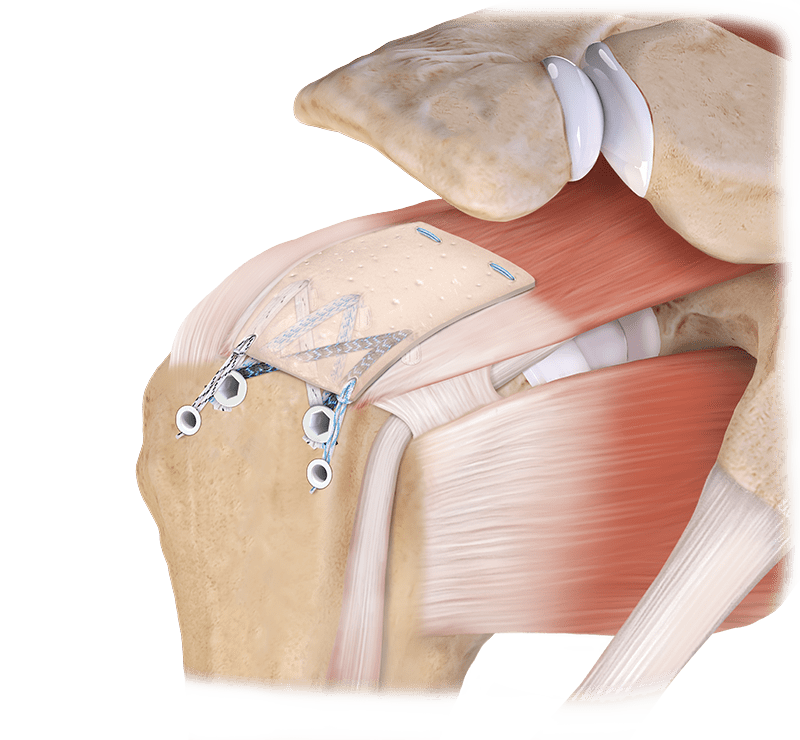
CuffMend™ Rotator Cuff Augmentation System Features and Benefits
Product Demonstrations | 02:35 | English | 10/02/2024 | AN1-000547-en-US A
CuffMend™ Rotator Cuff Augmentation: Second-Look Surgery at 6 Weeks
Misty Suri, MD
Case Presentation Videos | 04:31 | English | 01/31/2025 | VID1-006690-en-US A
CuffMend™ Surgical Technique Featuring FiberStitch™ RC
Patrick J. Denard, MD
Surgical Technique Videos | 05:43 | English | 05/29/2024 | VID1-004108-en-US B
CuffMend™ Rotator Cuff Augmentation Using the Autograft Tissue Compression System With a Biceps Tendon Autograft
Eugene Ek
Case Presentation Videos | 05:54 | English | 06/04/2025 | VID20-007248-en-AU A
CuffMend™ Rotator Cuff Augmentation Using Knotless SwiveLock® Anchors for Lateral Fixation
Stephen Brockmeier, MD
Surgical Technique Videos | 08:18 | English | 10/16/2024 | VID1-005866-en-US A
CuffMend™ Rotator Cuff Augmentation System
Surgical Technique Guides | English | 01/04/2024 | LT1-000201-en-US F
Videos
(22)
arrow_drop_down
Videos
Documents
(14)
arrow_drop_down
Documents
English
(36)
arrow_drop_down
Languages
Clear All Filters
Surgical Technique Videos (7)
Posterior-Approach CuffMend™ Augmented Rotator Cuff Repair With FiberStitch™ RC
Christopher T. Donaldson, MD
05:38 | English | 12/10/2024 | VID1-004077-en-US A
CuffMend™ Augmented Rotator Cuff Repair With FiberStitch™ RC Implant
Brian J. Cole, MD, MBA
06:39 | English | 12/03/2024 | VID1-004258-en-US A
CuffMend™ Rotator Cuff Augmentation With the Synergy Vision™ Imaging System and Nano Vision™ Feature
Misty Suri, MD
04:12 | English | 11/12/2024 | VID1-006282-en-US A
Graft Preparation for CuffMend™ Rotator Cuff Augmentation System
Thomas J. Kovack, DO
03:17 | English | 10/21/2024 | VID1-002384-en-US B
CuffMend™ Rotator Cuff Augmentation Using Knotless SwiveLock® Anchors for Lateral Fixation
Stephen Brockmeier, MD
08:18 | English | 10/16/2024 | VID1-005866-en-US A
Graft Preparation for CuffMend™ Posterior Approach
02:28 | English | 10/14/2024 | VID1-006070-en-US A
CuffMend™ Surgical Technique Featuring FiberStitch™ RC
Patrick J. Denard, MD
05:43 | English | 05/29/2024 | VID1-004108-en-US B
Surgical Technique Animations (1)
CuffMend™ Rotator Cuff Repair Augmentation System
03:04 | English | 02/07/2024 | AN1-000270-en-US D
Surgical Technique Guides (2)
CuffMend™-System zur Augmentation der Rotatorenmanschette
German | 12/15/2025 | LT1-000201-de-DE F
CuffMend™ Rotator Cuff Augmentation System
English | 01/04/2024 | LT1-000201-en-US F
Brochures (1)
New Product Spotlight - Shoulder Innovations | 2024
English | 01/31/2023 | evBR1-002463-en-US E
Case Presentation Videos (6)
CuffMend™ Rotator Cuff Augmentation System: My First 50 Cases
Matt Ravenscroft, FRCS, MBBS
04:07 | English | 10/21/2025 | VID1-007659-en-US A
Case Presentation: CuffMend™ Rotator Cuff Augmentation for a Partial-Thickness Tear
Nicholas Ramos, MD
05:07 | English | 10/07/2025 | VID1-007710-en-US A
CuffMend™ Rotator Cuff Augmentation Using the Autograft Tissue Compression System With a Biceps Tendon Autograft
Eugene Ek
05:54 | English | 06/04/2025 | VID20-007248-en-AU A
CuffMend™ Rotator Cuff Augmentation: Second-Look Surgery at 6 Weeks
Misty Suri, MD
04:31 | English | 01/31/2025 | VID1-006690-en-US A
My First 300 CuffMend™ Rotator Cuff Augmentation Cases
Misty Suri, MD
01:50 | English | 01/17/2025 | VID1-006689-en-US A
When to Consider CuffMend™ Augmentation in Patients With a Lower RoHI Score
Patrick J. Denard, MD
05:33 | English | 09/13/2024 | VID1-006527-en-US A
Catalogs (1)
Shoulder and Elbow Next Generation in Repair and Reconstruction
English | 02/02/2024 | LB1-0220-EN AE
Coding Guides (3)
CuffMend™ Rotator Cuff Repair Augmentation System 2026 Coding and Reimbursement Guidelines
English | 02/24/2026 | DOC1-002093-en-US A
SpeedFLEX™ Implant 2026 Coding and Reimbursement Guidelines
English | 02/13/2026 | DOC1-002121-en-US A
CuffMend™ Rotator Cuff Repair Augmentation System 2025 Coding and Reimbursement Guidelines
English | 07/01/2025 | DOC1-001694-en-US B
Office Forms & Reference Documents (1)
Rotator Cuff Repair with ArthroFlex Letter of Medical Necessity Template
English | 10/30/2025 | OF1-000439-en-US B
Presentation Videos (2)
Graft Augmentation of Repairable Rotator Cuff Tears: An Algorithmic Approach Based on Healing Rates
Patrick J. Denard, MD
09:16 | English | 08/29/2023 | VID1-003194-en-US A
Evidence for Rotator Cuff Augmentation With ECM
Brian J. Cole, MD, MBA
09:45 | English | 11/18/2021 | VID1-002713-en-US A
Product Demonstrations (1)
CuffMend™ Rotator Cuff Augmentation System Features and Benefits
02:35 | English | 10/02/2024 | AN1-000547-en-US A
Quick Facts (1)
Predictive Healing Score for Decision-Making
English | 07/03/2023 | DOC1-000787-en-US B
Scientific Updates (1)
Dermal Allograft Augmentation of Rotator Cuff Repair Scientific Update
English | 08/27/2024 | DOC1-000573-en-US E
SpeedPearls (5)
SpeedPearl: Opening the CuffMend™ Graft Spreader in a Tight Space
Patrick J. Denard, MD
00:26 | English | 01/05/2026 | VID1-007319-en-US B
SpeedPearl: Using the ApolloRF® i90 Probe for CuffMend™ Rotator Cuff Augmentation Technique
Asheesh Bedi, MD
00:58 | English | 10/08/2025 | VID1-007875-en-US A
SpeedPearl: Portal Placement for CuffMend™ Rotator Cuff Augmentation With FiberStitch™ RC
00:37 | English | 07/29/2024 | VID1-004099-en-US A
SpeedPearl: Alternative FiberStitch™ RC Fixation Method for CuffMend™ Rotator Cuff Augmentation Procedure
Patrick J. Denard, MD
00:35 | English | 05/23/2024 | VID1-004110-en-US A
SpeedPearl: CuffMend™ Lateral Portal Placement
Patrick J. Denard, MD
00:41 | English | 04/30/2024 | VID1-004109-en-US A
Surgeon Newsletters (5)
Scope This Out Volume 27, Number 1
English | 03/02/2026 | LN1-000660-en-US A
Scope This Out Volume 26, Number 2
English | 10/22/2025 | LN1-000646-en-US A
Scope This Out Volume 26 Number 1
English | 02/27/2025 | LN1-000623-en-US A
Scope This Out Volume 25, Number 01
English | 02/09/2024 | LN1-000570-en-US A
Scope This Out, Volume 23, Number 1
English | 03/14/2022 | LN1-000401-en-US A